The third day of the biomanufacturing bootcamp was dedicated to cell disruption and centrifugation. In smaller scale conditions these are processes that would normally not take an entire day, but we had a lot of bacteria that needed to be busted open. After all, that is where our green fluorescent protein (GFP) is!
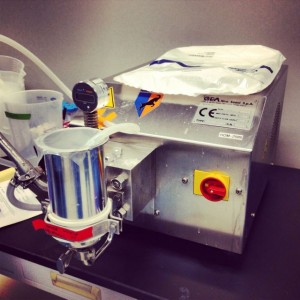
The GEA Niro Soavi homogenizer disrupts cells by generating incredible pressure and forcing the slurry through a small hole. When the cells come out the other side, the drop in pressure is so great that the cells explode. To ensure complete homogenization we ran the batch through the machine twice. Our homogenate (i.e., all the busted open bacteria) contains the precious GFP, but we need to separate it from all the other bacterial junk.
So, centrifugation was next. We used a disc stack centrifuge, which is a remarkable piece of engineering. Inside the conical dome there is a series of stacked discs that separate the liquid containing the protein of interest and the solids which are cellular debris. The disc stack has a large capacity so it can be continuously fed with homogenate. The exiting fluid is given a little back pressure to ensure no debris makes it through into the liquid product (supernatant).
Once processing was complete, the machine needed to be taken apart and cleaned. Inside the dome of the centrifuge we saw one of the end products of our homogenization and centrifugation steps — the leftover paste that represented the cellular guts of trillions of bacteria. We started with approximately 13 liters of bacteria liquid culture from the bioreactor and reduced that to enough cellular debris to fill a small ziplock sandwich bag. I was curious to see how much GFP was still in the paste (or pellet) that would be discarded so I ran a black light over it. It glowed green under the light so we know that we had some product loss at that step.
Now we have officially left upstream manufacturing and are ready to begin purifying our product. Almost every step has been very close to an industry process up to this point. However, It would be difficult for us to purify this quantity and still finish the class this week so we need to work with smaller volumes. The next step will involve strong anion-exchange chromatography to purify the GFP. We will need to dilute the supernatant we collected from today to reduce conductivity so our GFP will bind to the column. We will also need to adjust the pH and filter the supernatant prior to the chromatography step.
One thing I learned today was that no matter how new or sophisticated the equipment there are always peculiarities to get used to. For example, all the spinning parts on our centrifuge were reverse threaded to avoid the problem of the centrifuge loosening the fittings and screws during operation (which could be disastrous considering it spins incredibly fast). So, reverse threading makes sense from an engineering perspective but it is easy for newbies to forget while you are assembling or taking apart the centrifuge. We were warned several times about “galling” the stainless steel fasteners by going the wrong way. It was a little nerve-racking because some parts would cost over $4000 to replace!