Downstream processing typically begins after harvest of your cells from the bioreactor. In our case we needed to disrupt or break open our cells because green fluorescent protein (GFP), our product, is made inside the bacteria, not secreted externally. Internally produced proteins have a few more processing steps than externally excreted ones because you have to deal with all the debris and immunogenic material of the disrupted cells.
Purification operations are a critical part of a downstream processing because this is where the product is separated from all the other stuff that makes up bacteria. This step is what gets the product to fill and finish and into the hands of doctors and patients. Purification can occur with a variety of methods and technologies, but chromatography is often preferred because it has very high resolution. Our purification process workflow was the following: dilute the supernatant containing GFP from the previous centrifugation step to reduce conductivity, adjust pH to 8.2, syringe filter the product with 0.45 micron sterile filters, and then run strong anion-exchange chromatography using the AKTA Prime Plus system. (For a great animation of this process on the AKTA Prime Plus, go to this link: http://www.ncbionetwork.org/interactive-e-learning-tools-iet. You need to download the web player to view it.)
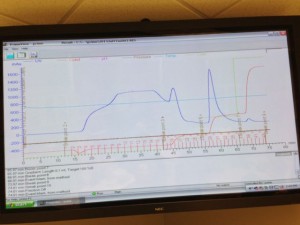
Anion-exchange chromatography uses resin inside a column that traps proteins based on their overall charge. Positively charged resin beads (we used a HiTrap Capto Q 5 column from GE) attract negatively charged proteins in low salt conditions. We first conditioned the column by running buffer through the system. Next, we loaded the GFP onto the column with a low salt buffer. This step is referred to as binding. Our chromatography system was programmed to run a step gradient increasing the salt conditions over time. Our elution buffer contained high salt concentration and was mixed with the starting buffer in increasing amounts. Under low salt conditions proteins other than GFP come off the column and are collected in the fraction collector. Once the salt concentration increased to a certain level, GFP came off the column and was collected. We could watch this happen with our black lights. In addition, the UV detector in the AKTA measured all protein coming off the column. We saw a very large peak near the end of our chromatogram, which represented the GFP coming off the column. We collected almost all the GFP in three fractions, although there was just a bit of tailing.
We now had highly purified GFP. We were told that this step typically yields over 80% purity. Ideally, we would have followed this step with hydrophobic interaction chromatography (HIC) and a desalting diafiltration to push the purity even higher, but we only had one day left for manual fill and labeling in the aseptic processing suite at BTEC.