In early July, in the midst of teaching my summer biomanufacturing course, the North Valley Biotechnology Center (NVBC) announced a two-week intensive cell culture and stem cell techniques course with Dr. Jason Bush at Fresno State University. I leapt at the opportunity and was selected to join five other students and faculty from around California to participate in the course.
My cell culture skills needed some brushing up, and I have never had the opportunity to work with stem cells. I previously worked in a neuro-oncology research lab doing cell culture and fluorescent in-situ hybridization (FISH) of cancer cell chromosomes. That was more than a few years ago now, so I would have a lot of re-learning to do. Aside from intellectual curiosity, I wanted to take this class because we are beginning to offer our Associates of Science (A.S.) degree classes in the biomanufacturing program at Laney College. This Spring we will offer a bioreactor cell culture and protein recovery class, and I wanted to be up-to-date with the latest cell culture technology.
Down to Basics
The class was designed to be mostly laboratory-based so we could get as much hands-on instruction as possible. We started with learning basic techniques of cell culture, such as thawing cells, freezing and cryopreservation of cells, changing media, passaging cells, cell counting, media formulations, and sterile technique. Our tissue culture (TC) room had two hoods and we also had access to advanced equipment at Fresno State’s RIMI facility.
We worked mostly with C2C12 and HEK293 cell lines. C2C12 is a mouse myoblast cell line and HEK293 is a human embryonic kidney cell line. We also cultured MDA-MB-231 cells, mouse embryonic fibroblast (MEF) cells, and J1 mouse embryonic stem cells (mESCs). As our instructor Dr. Bush noted, “If you can culture HEK293’s, you can culture just about anything.” The amazing thing was that even though almost all of us had little experience in culturing eukaryotic cells we did not have a single instance of contamination over the two weeks!
One thing that was unique about this class as compared to others I have taken is that we had the opportunity to practice each technique we learned multiple times. We had cultures running continuously throughout the two weeks and, as we quickly learned, you cannot leave them alone for more than a day before they demand your attention. Cultures need continuous care and monitoring to prevent overpopulation, starvation, and contamination. Certain cell lines will even start to form structures if you let them get too crowded. C2C12 cells will produce muscle proteins and form myotubes if left too long at high density. HEK293’s will form kidney tubules when packed too closely together.
Cell Culture Applications
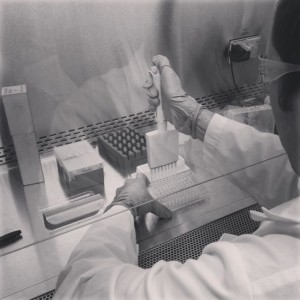
Setup of MTS assay for effect of two pesticides, toxaphene and methoxychlor, on breast cancer cells, MDA-MB-231.
Once we had gotten the basics down, we were able to move on to more advanced topics. Since contamination is a constant threat in cell culture, we learned how to do a mycoplasma test. There are various methods for testing for mycoplasma, such as complement fixation or DAPI staining. We went with a PCR mycoplasma detection kit. Testing for mycoplasma is routine in academic research and pharmaceutical production, so it was good to be exposed to this test.
Now that we could culture cells successfully and keep them free of contamination, we started doing things with our cells. The first application we learned was the cell-based assay. Cell-based assays are the true power of working in vitro. For our cell-based assay, we tested the effects of two pesticides, toxaphene and methoxychlor, on proliferation of a breast cancer cell line, MDA-MB-231. We used an MTS assay in which tetrazolium salt gets reduced into a purple product, formazan. The reduction is accomplished by the cell’s mitochondria, which makes the assay a good indicator of cellular proliferation.
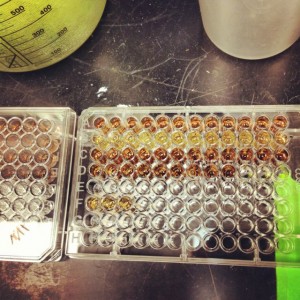
MTS assay results for the effects of toxaphene and methoxychlor on breast cancer cells (MDA-MB-231).
We ran the MTS assay on a 96-well dish. The first row is our control showing normal cellular proliferation. The second row is the highest concentration of toxaphene, and the third and fourth rows are successively lower concentrations. Clearly, these pesticides are toxic to cells but these are all at very high concentrations — high enough to cause organ failure and death if someone drank it. The point was to show the principle of a MTS assay. One could easily swap pesticides with a potential chemotherapy agent and test its effectiveness against this aggressive breast cancer cell line.
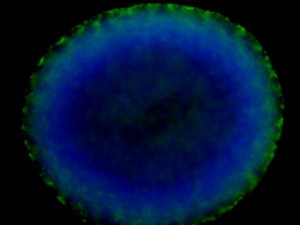
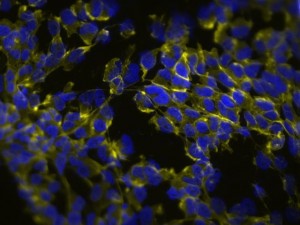
The next exciting application we learned was 3D cell culture. This new approach is gaining popularity in cell culture research because growing cells in three-dimensional space rather than on a flat, two-dimensional plate surface more accurately represents the in vivo condition. As our instructors put it, “Where do we have perfectly flat surfaces on our body?” We started with a gel-based culture using 20% methocel and our HEK293’s grown in a standard flask. The procedure was much like a regular cell culture except we used a 96-well plate to form our spheroids. It was easy to tell if a spheroid culture had grown in a well because they were large enough to see with the naked eye. To visualize the structures better, we fixed the cells with paraformaldehyde and stained them with DAPI and phalloidin 488, which interestingly comes from the death cap mushroom that grows in the Bay Area. We also stained 2D cultured HEK293’s for comparison. The images we generated were astounding — easily the best fluorescent microscope images I have ever made!
Spheroids are very interesting structures. Certain cell lines will form them naturally if given the right supporting matrix. Many studies have shown how gene expression changes in 3D cultures versus 2D. Other factors such as cell polarity, growth, morphogenesis, motility, and differentiation differ in 3D versus 2D cultures. One thing that really fascinated me was that 3D culturing of cells causes cellular differentiation without the use of inducing factors. Spheroid bodies naturally form and the cells within them differentiate to form more complex extra cellular matrix (ECM). The center of the spheroid bodies will become necrotic and hollow, as can be faintly seen in the fluorescent microscope image.
There are some disadvantages to 3D cultures. They vary in their ability to mimic in vivo conditions. Some experimental techniques use scaffolding and co-cultures to get closer to in vivo conditions. Some day soon it may be possible to introduce vasculature and transport of small molecules. Microfluidic devices can achieve some of this now. Another disadvantage is that 3D cultures tend to model static or short-term conditions, which of course is not how in vivo systems work.
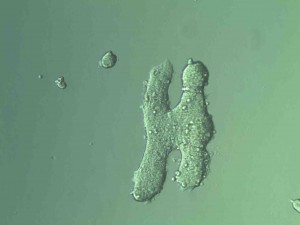
One of the most popular ways to get a 3D culture going is the hanging drop method. The name says it all: cells are literally suspended in a drop on the underside of a petri dish lid. We utilized this technique with our HEK293 cell cultures. It was a lot of fun to set up, although the final step of inverting the lid over the petri dish was a little nerve-racking because it is very easy to jiggle the lid too much and ruin the perfect dome shape of the drops. We checked these a few days later and found spheroid structures growing in our hanging drops. One of the cultures I transferred to a dish randomly formed a letter “H” (picture at end).
Stem Cell Techniques
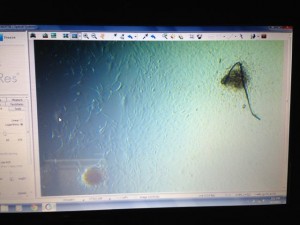
Next, we were on to stem cells. Like I said earlier, I have never had the chance to work with stem cells so I was looking forward to this part of the course. We cultured mouse embryonic stem cells, specifically the J1 line. J1 cells were first grown in the lab in 1981 and have proved to be a useful model to study embryonic development and cell fate. Stem cells will naturally differentiate when grown in culture so Leukemia Inhibitory Factor (LIF) is added to maintain cultures in an undifferentiated state. (By the way, LIF is quite expensive. Only one company, Millipore EMD, supplies LIF and it costs over $1000 for a million units!) Also, stem cells do best when grown on a feeder layer. We treated our mouse fibroblast feeder layer cells with mitomycin C, which is highly toxic to cells and prevents them from dividing. Technically, stem cells grown on a feeder layer represent a co-culture since more than one cell line is in the same culture. We ended up getting some nice J1 embryoid bodies (EBs) forming on our feeder layer, as can be seen in the photo of the microscope computer screen. We had planned to coax these cells into the cardiac lineage using calcium treatments, but we had some difficulty passaging the EBs so we did not complete the task before the end of the second week. The problem was that our EBs were sticking to bacteriological plates and we did not know why. It was too bad, but the reality of working in the laboratory is that not everything works out!
One thing we did use our embryoid bodies for was an enzyme-labeled fluorescence (ELF) assay. This assay measures endogenous phosphatase activity, which is correlated to pleuripotency in stem cells. Thus, the brighter the EBs appear the more undifferentiated they are. We had some trouble with detection using our Zeiss Axio Imager A1 and it may have been due to using the wrong filter. The workaround was to do a time series of the EBs to see the change in fluorescence. The image to the right shows an EB at the end of the time series.
Flow Cytometry
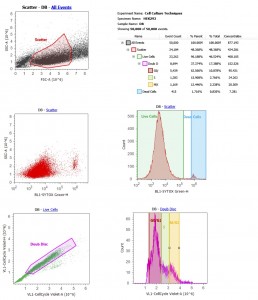
Nearing the end of the second week, we had time to learn one more advanced technology: flow cytometry. Flow cytometry uses lasers to count, sort, and detect proteins or biomarkers in cells. It relies on fluorescence and a variety of exclusion dyes (e.g., DAPI, Trypan blue, membrane dyes, etc.) and antibody stains. Fresno State’s RIMI facility has a Life Technologies Attune Flow Cytometer, which uses sound instead of fluid pressure to focus cells. We treated our HEK293 cells with two basic stains: Sytox Green and DyeCycle Violet. We ran our samples on the flow cytometer and set it to count 50,000 events. Once the data was collected, we got a crash course in gating and interpretation of results. What did we find? Well, this was not an experiment so we were just looking at untreated HEK293 cells in culture. DyeCycle Violet allowed us to see a distribution of cells in the cell cycle. As you can see from my results to the right, most of my cells were not dividing (bottom right graph), probably because they were over confluent. We did see interesting differences in cell cycle distribution depending on whose culture was being analyzed.
Give Me an H, Vasily. One H Only, Please.
What an incredible two weeks! I am amazed at all Dr. Bush and his graduate students were able to cover in such a short time. Plus, we spent the majority of it working in the laboratory, rather than sitting in lecture. It was one of the most hands-on classes I have ever taken. I gained a lot of confidence in my cell culture technique. If someone handed me a cryovial of adherent cells, I know I could culture them and keep them free of contamination. I am looking forward to taking this knowledge back to the biomanufacturing program at Laney College.
A special thanks is in order to Dr. Heather Brown at the North Valley Biotechnology Center for organizing this class. I hope NVBC offers more classes like this in the future!